You will first need to calculate the moles of carbon dioxide. Then write a balanced equation and use the mole ratio between carbon dioxide and ammonium carbonate ((NH4)2CO3) to determine the moles of (NH4)2CO3 required. Then calculate the mass of (NH4)2CO3 required from moles and molar mass. Convert 9.52 g of CO2 to moles.hi..formula ion for ammonium ion is= NH4^+(4 is subscript for lower and + is subcript for charge) and for oxygen ion is=O^2- (2- is subscript for charge) thus u just cross THE CHARGE ONLY. NH4^+ O2-the charge + 2-x(cross THE CHARGE) u will get (NH4)2OMolar mass of *3(NH4)2O is 156.2290 g/mol Convert between *3(NH4)2O weight and molesOxygen is O2- Electronic configuration of oxygen is 2.6, and that's how the symbol for Oxygen becomes O 2- as it requires 2 more electrons to fill up the second shell. Need to duplicate another NH4+ to balance the charge. As such the symbol is for ammonium oxide is (NH4)2OAmmonium phosphate ((NH4)2(HPO4)) diazanium hydrogen phosphate. diazanium;hydrogen phosphate (NH4)2HPO4. AI3-25349. Ammonium hydrogen phosphate solution. di-ammonium phosphate. Phosphoric acid, ammonium salt (1:2) ACMC-1BBLK. DAP, DAPLG. EC 231-987-8. KSC377C4F. Diammonium Phosphate Food Grade. DTXSID6029705. CTK2H7142. KS-000011AS
What is the chemical formula for Ammonium oxide? | Yahoo
What is the molar mass of (NH4)2O? Explain how you calculated this value.? Chem Help Please. Answer Save. 3 Answers. Relevance =D. 8 years ago. Favorite Answer. Molar Mass is grams over moles. Use the periodic table to find the molecular mass of each element and add them up, that is the amount of grams in one mole of this compound.The ammonium ion that results from this neutralization has its own equilibrium (Ka): NH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq) *this makes the solution slightly more acidic. Acid-Base Indicators. The equivalence point in a titration can be estimated with the use of an acid-base indicator.This program was created with a lot of help from: The book "Parsing Techniques - A Practical Guide" (IMHO, one of the best computer science books ever written.); The Gold Parsing System (Hats off! What a great software product!) The Calitha - GOLD engine (c#) (Made it possible for me to do this program in C#)The formula mass of an ionic compound is the mass, in grams, of one mole of the formula unit. To find this value, find the sum of the mass of each atom in the formula.
Molar mass of *3(NH4)2O
Ammonium Oxide (NH4)2O Molar Mass, Molecular Weight. Molar Mass: 52.0763In this video we'll write the correct formula for Ammonium oxide, (NH4)2O.To write the formula for Ammonium oxide we'll use the Periodic Table, a Common IonTop contributors to the provenance of Δ f H° of [NH4]+ (aq) The 16 contributors listed below account for 90.6% of the provenance of Δ f H° of [NH4]+ (aq). Please note: The list is limited to 20 most important contributors or, if less, a number sufficient to account for 90% of the provenance.Koktaite ((NH4)2Ca(SO4)2.H2O) | CaH12N2O9S2 | CID 6337632 - structure, chemical names, physical and chemical properties, classification, patents, literatureMolar mass calculator computes molar mass, molecular weight and elemental composition of any given compound.
Molar mass of (NH4)2O = 52.07632 g/mol
Convert grams (NH4)2O to moles or moles (NH4)2O to grams
Molecular weight calculation:(14.0067 + 1.00794*4)*2 + 15.9994
Element Symbol Atomic Mass # of Atoms Mass PercentHydrogen H 1.00794 8 15.484%Nitrogen N 14.0067 2 53.793%Oxygen O 15.9994 1 30.723%In chemistry, the method weight is a amount computed via multiplying the atomic weight (in atomic mass units) of each and every part in a chemical components through the collection of atoms of that part present in the method, then including all of those products together.
Using the chemical components of the compound and the periodic desk of components, we will be able to upload up the atomic weights and calculate molecular weight of the substance.
Finding molar mass begins with units of grams in keeping with mole (g/mol). When calculating molecular weight of a chemical compound, it tells us what number of grams are in one mole of that substance. The formulation weight is solely the load in atomic mass units of all of the atoms in a given system.
Formula weights are especially helpful in determining the relative weights of reagents and merchandise in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
If the components used in calculating molar mass is the molecular formula, the components weight computed is the molecular weight. The proportion by weight of any atom or workforce of atoms in a compound can also be computed through dividing the whole weight of the atom (or group of atoms) in the formula by means of the components weight and multiplying by way of 100.
The atomic weights used in this web site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is tips on how to calculate molar mass (reasonable molecular weight), which is in keeping with isotropically weighted averages. This isn't the similar as molecular mass, which is the mass of a unmarried molecule of well-defined isotopes. For bulk stoichiometric calculations, we are typically determining molar mass, which can also be referred to as standard atomic weight or average atomic mass.
A common request on this site is to convert grams to moles. To complete this calculation, it's important to know what substance you are trying to transform. The reason why is that the molar mass of the substance impacts the conversion. This web page explains how one can find molar mass.
Writing Formulas
Ammoniomagnesiovoltaite (NH4)2Mg5Fe 3Al(SO4)12•18H2O
Uptake Coefficients For N 2 O 5 On Ammonium Sulfate, Ammo- Nium... | Download Table

Effect Of The Variation Of The Feed Time Of The Biological Reactor... | Download Scientific Diagram

Study Of Thermal Decomposition Of Ammonium Paratungstate
Study Of Thermal Decomposition Of Ammonium Paratungstate
Godovikovite (NH4)(Al, Fe3+)(SO4)2
PS1 CHE213 | Sets Of Chemical Elements | Atoms

Solved] Its Urgent. The Oxidation Of NH4+ To NO3- In Acid Solution PH=5.600 Is Described By The Following Equation: NH 4 + (aq)+2O 2 (aq)+H 2 O(l)-... | Course Hero
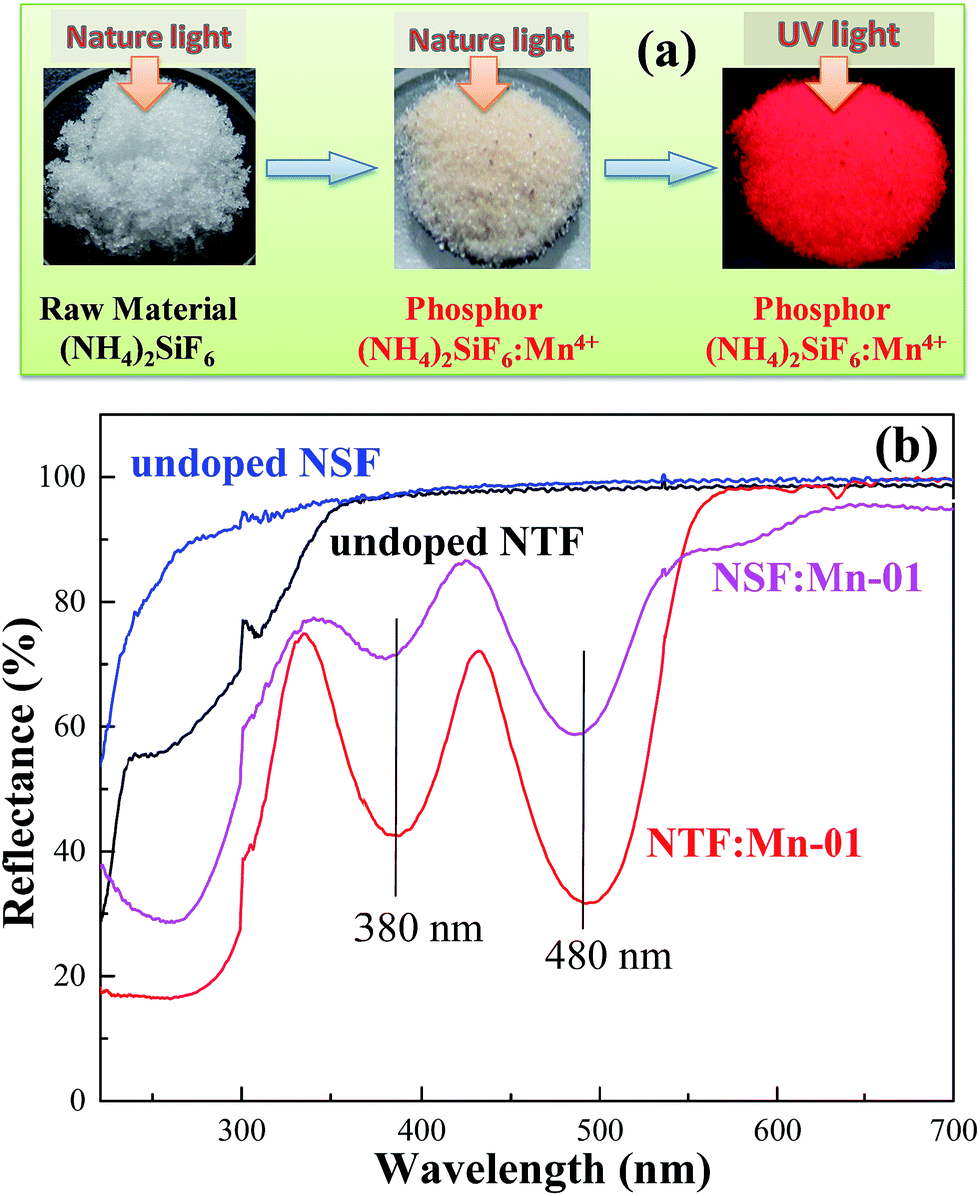
Mn4+ Doped (NH4)2TiF6 And (NH4)2SiF6 Micro-crystal Phosphors: Synthesis Through Ion Exchange At Room Temperature And Their Photoluminescence Properties - RSC Advances (RSC Publishing)
Solved: Blem 6.43 Correct Part C Write The Formula For The... | Chegg.com

Processes | Free Full-Text | Fabrication Of Ultrathin MoS2 Nanosheets And Application On Adsorption Of Organic Pollutants And Heavy Metals | HTML

Beshtauite (NH4)2(UO2)(SO4)2·2H2O
Identification Of Hydrogen Species In Alunite-type Minerals By Multi-nuclear Solid-state NMR Spectroscopy | SpringerLink

Chemistry 30
Untitled
NH4)HCO3 (Ammonium Carbonate)

Characterization Of Ammonioleucite (NH4)[AlSi2O6] And ND4-ammonioleucite (ND4)[AlSi2O6] Using IR Spectroscopy And Rietveld Refin
Magnetic Field-temperature Phase Diagram Of Multiferroic (NH 4 ) 2 FeCl 5 ·H 2 O | Npj Quantum Materials

A Facile One-step Synthesis Of Super-hydrophilic (NH4)0.33WO3/WS2 Composites: A Highly Efficient Adsorbent For Methylene Blue - New Journal Of Chemistry (RSC Publishing)

0 comments:
Post a Comment